Bio-compatible Fasteners in Orthopedic Surgery: Magnesium-Based Screw Degradation Rates in Bone Healing
Executive Summary
The orthopedic implant industry is undergoing a paradigm shift from bio-inert permanent fixations to bio-resorbable metallic systems. At the forefront of this evolution is the use of magnesium (Mg) and its alloys for surgical fasteners. Unlike traditional titanium or stainless steel implants, which often require a secondary retrieval surgery and can cause stress-shielding, magnesium-based screws offer a mechanical modulus close to human cortical bone. This white paper explores the critical intersection of degradation kinetics and physiological bone healing. We analyze the biochemical pathways of magnesium resorption, the impact of alloying elements on corrosion rates, and the clinical outcomes of modern fasteners, with a particular focus on market leaders such as Bioretec Oy (Nasdaq Helsinki: BRETEC).
1. Introduction: The Evolution of Orthopedic Fixation
For decades, the “gold standard” for fracture fixation involved stainless steel and titanium alloys. While these materials provide excellent mechanical stability, their permanent presence within the human body introduces long-term complications. These include chronic inflammation, localized osteopenia due to stress-shielding, and the psychological and physical burden of secondary surgeries for implant removal. In pediatric orthopedics, the challenges are even more acute, as permanent hardware can interfere with natural bone growth.
The emergence of bio-compatible, degradable metals has addressed these systemic issues. Magnesium is the fourth most abundant mineral in the human body and is naturally sequestered in bone tissue. Its density (1.7–2.0 g/cm³) and elastic modulus (40–45 GPa) are remarkably similar to those of cortical bone (15–25 GPa). This mechanical synergy allows for a more natural distribution of load across the fracture site, stimulating osteoblast activity through “Wolff’s Law” and accelerating the healing process.
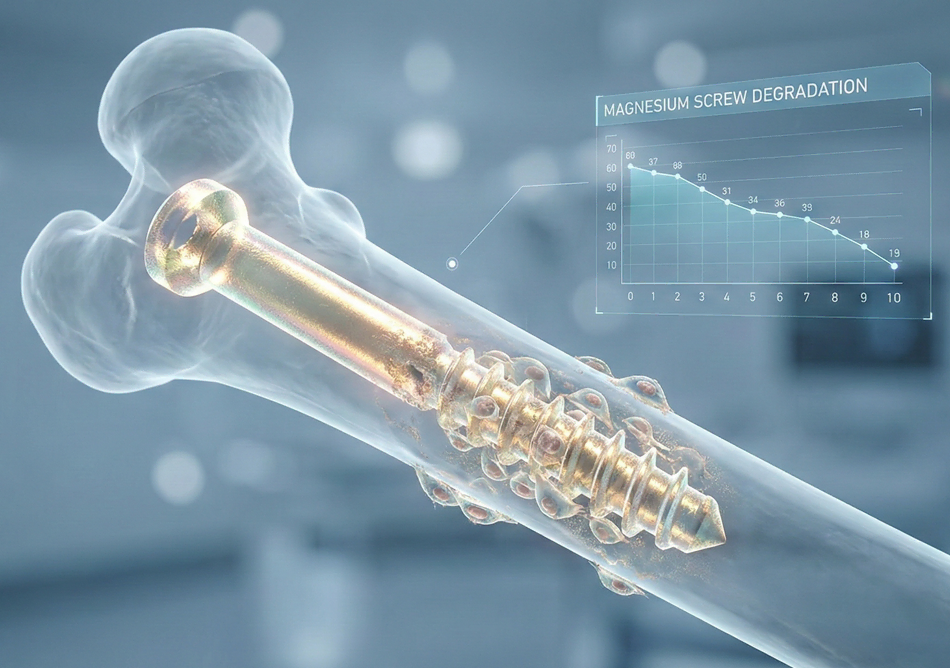
2. The Biochemistry of Magnesium Degradation
Magnesium is highly reactive in aqueous, chloride-rich environments like human physiological fluid. The degradation process is essentially a corrosion reaction that produces magnesium hydroxide and hydrogen gas. The fundamental chemical reaction is expressed as:
In the body, the magnesium hydroxide layer () provides a temporary buffer, but it is quickly converted into highly soluble magnesium chloride by interacting with chloride ions in the blood. This results in the release of ions, which are either utilized by the surrounding bone for mineralization or excreted via the kidneys. The primary challenge in magnesium-based screw design is managing the rate of this reaction to ensure the screw maintains structural integrity until the bone has achieved “clinical union.”
2.1 Hydrogen Gas Evolution and Management
A notable byproduct of magnesium degradation is hydrogen gas (). In early clinical trials, rapid degradation led to the formation of large gas pockets, which could delay healing or cause subcutaneous emphysema. Modern alloy engineering and surface treatments (such as micro-arc oxidation) have significantly mitigated this. By controlling the corrosion rate to below 0.5 mm/year, the body can absorb the hydrogen gas at a rate equal to or faster than its production, eliminating the risk of gas-related complications.
3. Degradation Rates vs. Bone Healing Timelines
The efficacy of a bio-resorbable screw is defined by its “degradation profile.” The fastener must provide rigid stabilization during the initial inflammatory and repair phases of bone healing (typically 6–12 weeks) and then lose its mechanical strength only as the callus matures into lamellar bone.
3.1 The Three Phases of Resorption
- Phase 1: Initial Stability (0–6 Weeks): The screw remains 95-100% intact. Surface coatings prevent early corrosion, ensuring the fracture gap is maintained under physiological load.
- Phase 2: Gradual Fragmentation (6–24 Weeks): As bone bridging occurs, the screw begins to lose volume. Micro-cracks form within the alloy, but the surrounding new bone (callus) increasingly shares the load.
- Phase 3: Complete Resorption (12–24 Months): The metallic structure is entirely replaced by trabecular bone. Radiographic studies of magnesium screws often show “radiolucent zones” that gradually fill with high-density bone, indicating active osseointegration.
3.2 Impact of Alloying Elements
Pure magnesium degrades too quickly for most orthopedic applications. To tailor the degradation rate, engineers use specific alloying elements:
| Element | Function | Impact on Degradation |
|---|---|---|
| Zinc (Zn) | Refines grain structure | Slows corrosion, increases strength |
| Calcium (Ca) | Improves bio-compatibility | Accelerates bone mineralization |
| Yttrium (Y) | Heat resistance / Stability | Significantly reduces hydrogen evolution |
| Zirconium (Zr) | Grain refinement | Improves mechanical ductility |
4. Clinical Study Analysis: Magnesium Screws in Small Bone Fixation
Recent clinical trials have focused on the use of magnesium-based headless compression screws in distal radius fractures and hallux valgus (bunion) corrections. A 2024 longitudinal study compared magnesium-alloy (MgYREZr) screws against traditional titanium screws. The results indicated that while titanium screws remained unchanged, the magnesium screws showed a 25% reduction in volume at the 12-month mark with no loss of fixation. More importantly, patients in the magnesium group reported lower long-term pain scores, attributed to the absence of “cold-weather ache” and soft tissue irritation common with permanent metal hardware.
5. Market Leadership: Bioretec Oy and the RemeOs™ Platform
The commercialization of bio-resorbable metals is led by a few specialized firms. Bioretec Oy (Nasdaq Helsinki: BRETEC) has emerged as a pioneer with its RemeOs™ technology. Their magnesium-based trauma screws are designed to be “implanted and forgotten,” a slogan that highlights the elimination of hardware removal procedures.
Bioretec’s approach utilizes a proprietary alloy that optimizes the balance between initial tensile strength and long-term resorption. In March 2023, the company received FDA De Novo clearance for its RemeOs™ trauma screw, marking a historic milestone as the first bio-resorbable metal implant authorized for the U.S. market. This regulatory validation has catalyzed interest in the stock (BRETEC), as it positions the company to disrupt the multibillion-dollar orthopedic trauma market currently dominated by giants like Stryker and DePuy Synthes.
6. Challenges and Future Perspectives
Despite the advantages, magnesium-based fasteners face hurdles. The “anodic” nature of magnesium makes it susceptible to rapid localized corrosion in highly acidic environments (e.g., infected tissue). Furthermore, the imaging of these screws under MRI can sometimes produce minor artifacts, though significantly less than those produced by stainless steel.
The next frontier in this research involves “Smart Fasteners”—magnesium screws embedded with bioactive molecules or growth factors (like BMP-2) that are released at specific stages of the degradation process. This would transition the screw from a passive structural component to an active therapeutic agent.
7. Conclusion
Magnesium-based screws represent the pinnacle of “bio-mimetic” engineering in surgery. By matching the mechanical properties of bone and offering a controlled, safe degradation path, they eliminate the “dead weight” of permanent implants. As clinical data continues to demonstrate the safety and osteogenic benefits of these materials, and as companies like Bioretec Oy scale their global distribution, bio-resorbable metals are poised to become the standard of care for fracture fixation by the end of the decade.
Share This Story, Choose Your Platform!
